No Place to Hide: Navigating State Drug Price Transparency For Manufacturers
The rising tide of drug price transparency laws and what they mean for the future of pharma pricing compliance
For pharma manufacturers, state drug price transparency management is no longer just a policy issue — it’s a business imperative.
As states roll out legislation demanding greater visibility into drug pricing practices, you face mounting pressure to justify costs, disclose methodologies and align with evolving regulatory expectations. What was once considered proprietary is now under the spotlight. In this blog, we explore how pharma price transparency is reshaping manufacturer responsibilities, the strategic value of proactive compliance and what lies ahead in this rapidly changing landscape.
Beyond the sticker price: Defining pharma price transparency and state reporting
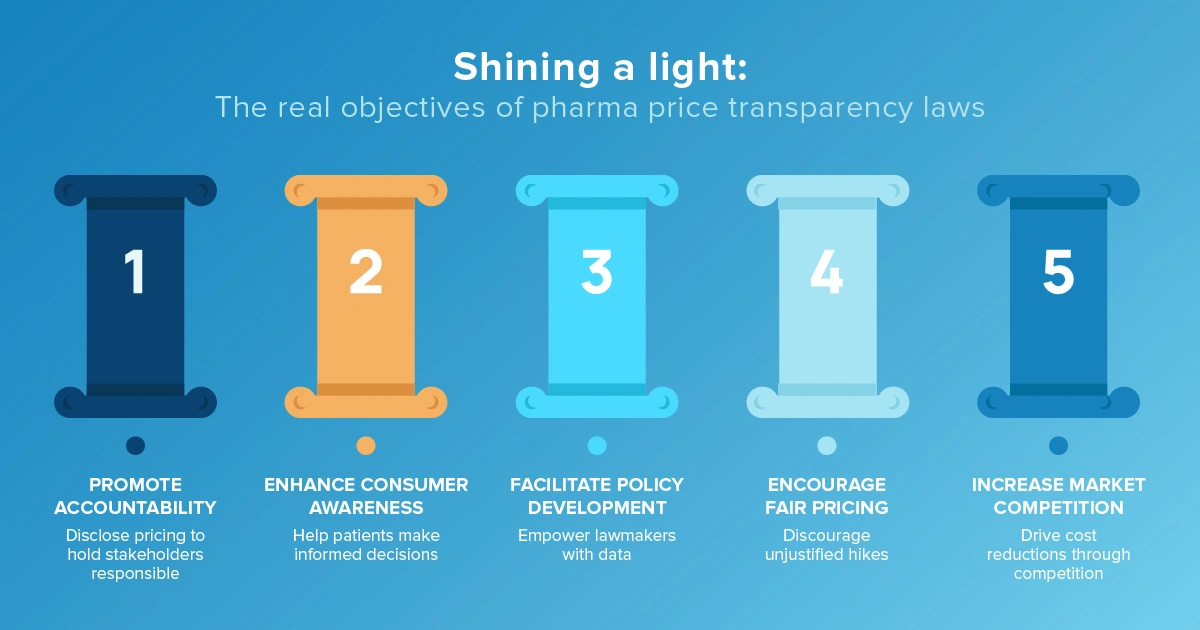
Pharmaceutical price transparency is the practice of making drug prices publicly accessible. It stems from the belief that greater openness in pricing can lead to more informed decisions by patients, healthcare providers and policymakers.
Vermont was the first state to enact a state drug price transparency law in 2016, requiring manufacturers to justify significant price increases. This pioneering move sparked a nationwide push for legislation aimed at demystifying drug pricing.
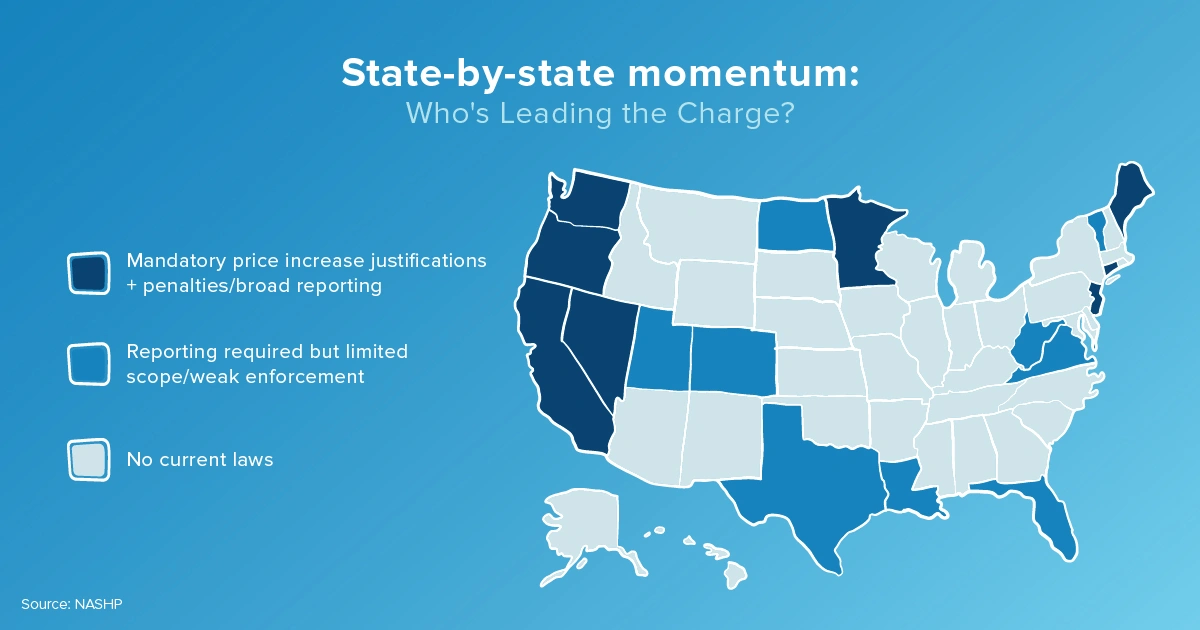
State drug price transparency management: Who’s leading the charge?
Since Vermont's initial step, several states have followed with their own regulations, creating a patchwork of state price reporting rules that pharma manufacturers must navigate:
Each state's approach varies, with some focusing on specific drugs or entities within the supply chain based on what the state spends on pharmaceuticals, others concentrating on higher-priced drugs, and others looking at pricing movements more broadly and tying price to cost.
“Transparency” is no longer optional — it’s a moving target that pharma must aim to meet.
Behind the curtain: Why true drug price transparency remains elusive
These are all tremendous goals, but they remain difficult to achieve fully. The public price, known as the Wholesale Acquisition Cost (WAC), is often arbitrary and detached from the actual cost of the drug. It is designed more for transactional convenience than true transparency. Obtaining true contract prices or rebate data is nearly impossible since those numbers are typically confidential. Even net prices are only estimations, not hard figures.
Add in the complex R&D costs, royalties and overlapping drug development processes, resulting in an industry cloaked in ambiguity. On the consumer side, patients rarely control what drugs are stocked at their pharmacy or how much their insurance plan pays. Price transparency without purchase power only goes so far. This makes drug price reporting compliance especially challenging.
Pharma price transparency laws: Clear intentions, cloudy results
While transparency aims to clarify drug pricing, it can inadvertently create confusion. Patients often lack the context to understand pricing decisions, especially with opaque discounts and rebates at play. Education must accompany disclosure for transparency to have a meaningful impact.
Pharma manufacturer pushback on state drug price transparency reporting
Pharma manufacturers are concerned that disclosing pricing data may reveal proprietary strategies or disrupt their competitive positioning. Some worry about being penalized in global pricing negotiations.
Overcoming these concerns requires regulatory guardrails and assurance that drug price transparency doesn’t compromise innovation or IP.
Fueling progress: Policies, partnerships and pharma compliance software
Achieving true drug price transparency demands a threefold strategy: robust regulation, strong collaboration and modern technology.
- Effective transparency hinges on clear, enforceable policies. States must implement standardized frameworks that reduce ambiguity and ensure consistency in collecting, formatting and reporting drug pricing data. Without a solid regulatory foundation, compliance becomes fragmented and burdensome.
- Collaboration across the healthcare ecosystem is critical. Everyone plays a role in building a transparent environment, from manufacturers and PBMs to providers, policymakers, and patients. Multi-stakeholder engagement reduces the reporting burden, drives alignment and ultimately enhances data quality.
- Digital innovation is the catalyst that connects it all. Technology-enabled pricing and compliance platforms streamline price reporting, automate state-specific requirements and provide real-time visibility to all stakeholders. When paired with strong policies and active partnerships, tech becomes a powerful enabler of transparency.
The road ahead: State drug price transparency and reporting compliance
Pharmaceutical price transparency isn’t just a trend—it’s an evolving mandate. The benefits are clear: more informed consumers, more competitive pricing and greater trust in the system.
But to get there, you and the industry must embrace collaboration, invest in tools and continue to innovate. There’s work to do, but the destination is worth the journey.
Navigating state drug price transparency compliance with software solutions
Our software solutions are purpose-built and provide an all-in-one, comprehensive platform to help you meet evolving state drug price reporting needs. With built-in rule logic and automated reporting formats, our revenue management platform simplifies pharma price transparency compliance and drives efficiency.
Take the pharma compliance and price transparency journey with us! Watch our webinar, where our industry experts will provide a strategic roadmap for winning at state drug price transparency with the right tools and processes needed to stay ahead of evolving regulations.
Get the latest news, updates, and exclusive insights from Vistex delivered straight to your inbox. Don’t miss out—opt in now and be the first to know!